What is Ectoin®?
Ectoin® is a low-molecular-weight, cyclic amino acid derivative produced by many different extremophilic microorganisms. Ectoin® belongs to the class of compatible solutes also called extrapolates (osmolytes from extremophiles) and was first isolated by Galinski and colleagues from the bacterium Ectothiorhodospira halo-Chloris found in Wadi Natrun, Egypt.
In extremophilic microorganisms, these low-molecular-weight compounds are accumulated in response to increased extracellular salt concentrations and other environmental changes, such as increased temperature. This is one of the ingenious strategies these organisms have developed to cope with harsh environmental conditions.
Extremolytes minimize the denaturation of biopolymers that usually occur under water stress conditions and are compatible with the intracellular machinery at high (>1M) concentrations. Extremolytes have a wide range of applications because they protect biological macromolecules and cells from damage by external stresses.

Ectoin® Comes from Extreme Environments, Protecting Microbial Survival
Extremophiles are a group of microorganisms that thrive in extreme environments, such as extreme cold, heat, acidity, alkalinity, salinity, and high pressure. Such bacteria often develop unique physiological characteristics to adapt to these extreme conditions.
How extremophiles survive in extreme environments relies heavily on their unique cell structures. Thermophiles are the most common group of extremophiles, and the cell membrane, which is vital for maintaining cell integrity and growth, exhibits different lipid compositions compared to normal bacteria in high-temperature bacteria.
The Asia-Pacific Rim volcanic belt has many geothermal hot spring areas on islands, including submarine hydrothermal vents, alkaline or acidic geothermal spring areas, and other extreme environments. Previous studies have revealed the existence of numerous high-temperature microorganisms in these natural hot environments.
Ectoin®, an extremolyte molecule, protects extremophiles. Derived from an original strain discovered on the Bonaire salt flats in the Caribbean by the German pharmaceutical company Bitop AG, this molecule is produced using patented biNEXT technology to ensure the highest quality natural Ectoin® molecules. It is used to treat respiratory diseases, allergies, and skin conditions. Ectoin® represents a widely applicable and well-tolerated natural protective molecule that can withstand harmful environmental influences like heat, drought, or ultraviolet radiation.

Characteristics of Ectoin®
1.Stabilisation of Proteins and the structure of cells
Ectoin® stabilizes biomolecules via a physical mechanism called “preferential exclusion.” According to this theory, the protein stabilization effects of osmolytes like Ectoin® are due to their effect on the solvent water. This leads to a preferential exclusion of the osmolyte from the protein surface and preferential hydration of the protein.
Because the surface area of globular proteins in the native state is smaller than in the denatured state, the equilibrium is shifted to the native state, stabilizing the native structure.
The osmolyte promotes the formation of water molecules in clusters. Thus, the exclusion hypothesis attributes the stabilizing effect of Ectoin® to changes in the surrounding water structure.
Ectoin® is, in contrast to sodium chloride, a strongly kosmotropic (water structure-forming) substance. An investigation of the oxygen radial distribution function of pure water, sodium chloride, and Ectoin® proved the stabilizing effect of Ectoin® on the water structure.
Figure 1,2,3: Effect of Ectoin® on the water structure
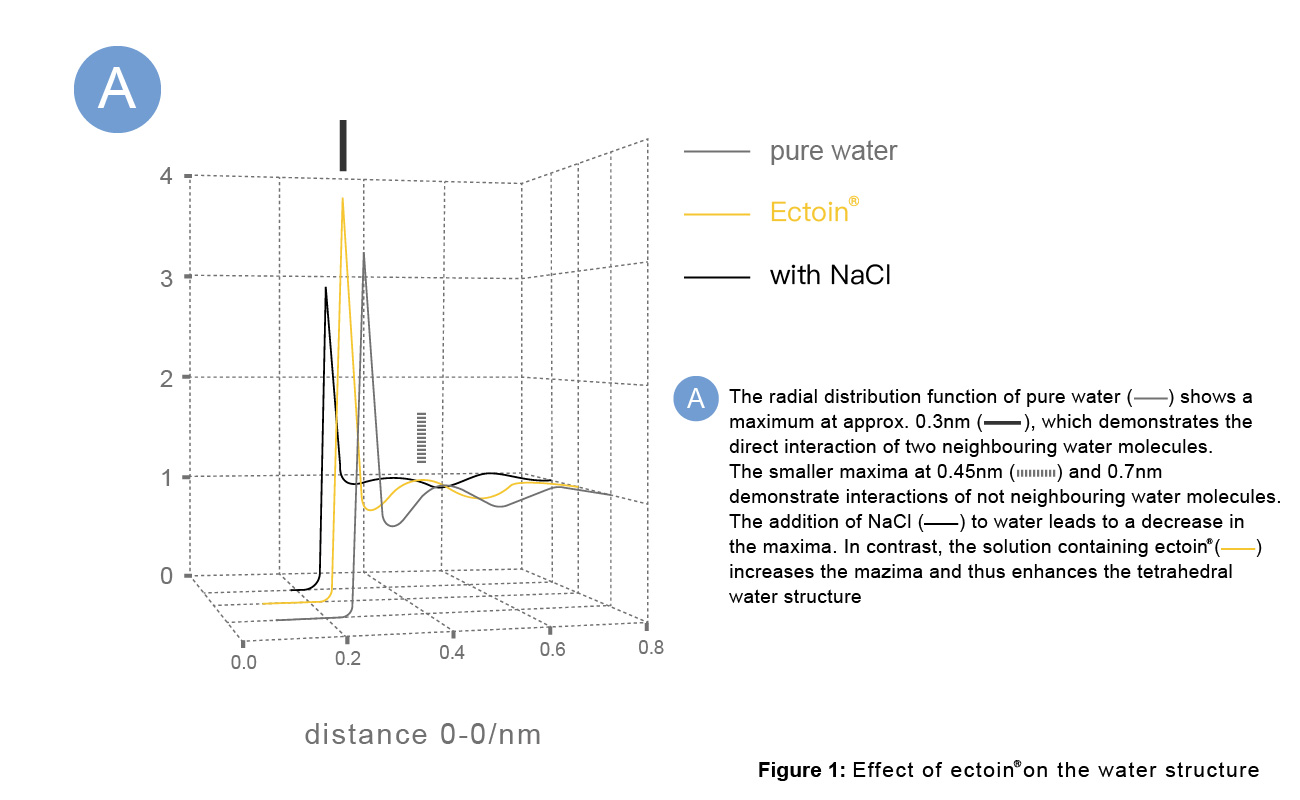
A: The radial distribution function of pure water shows a maximum of approx. 0.3nm, which demonstrates the direct interaction of two neighboring water molecules. The smaller maxima at 0.45nm and 0.7nm demonstrate interactions of water molecules that are not neighboring.
The addition of NaCl to water decreases the maxima. In contrast, the solution containing Ectoin® increases the maxima and thus enhances the tetrahedral water structure.
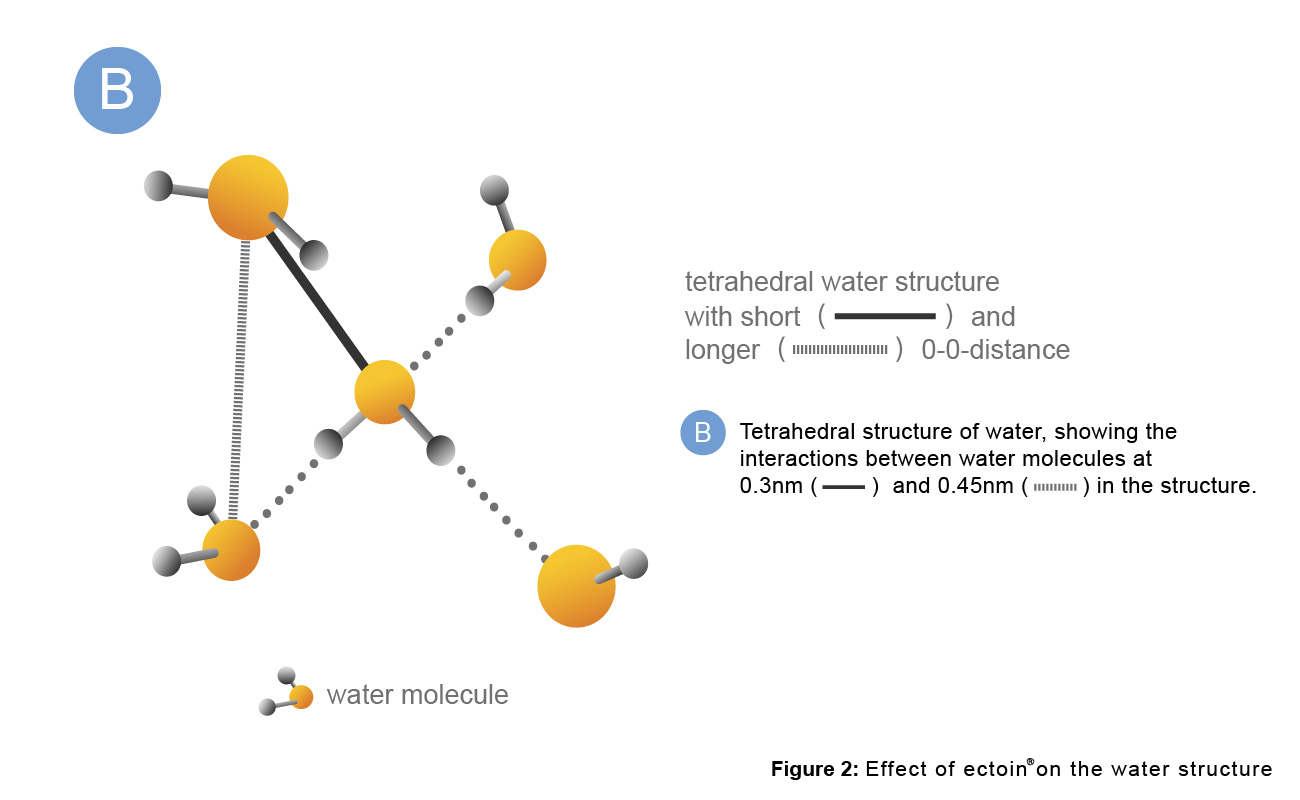
B: The tetrahedral structure of water shows the interactions between water molecules at 0.3nm and 0.45nm.

C: Complex formed by Ectoin® and water molecules.
Sodium chloride diminishes the interaction between water molecules. It destroys the water structure and is, therefore, chaotropic.
In contrast, a solution containing Ectoin® increases the number of neighboring water molecules. Thus, Ectoin® enhances the water-water interactions. Ectoin® stabilizes the tetrahedral structure of water.
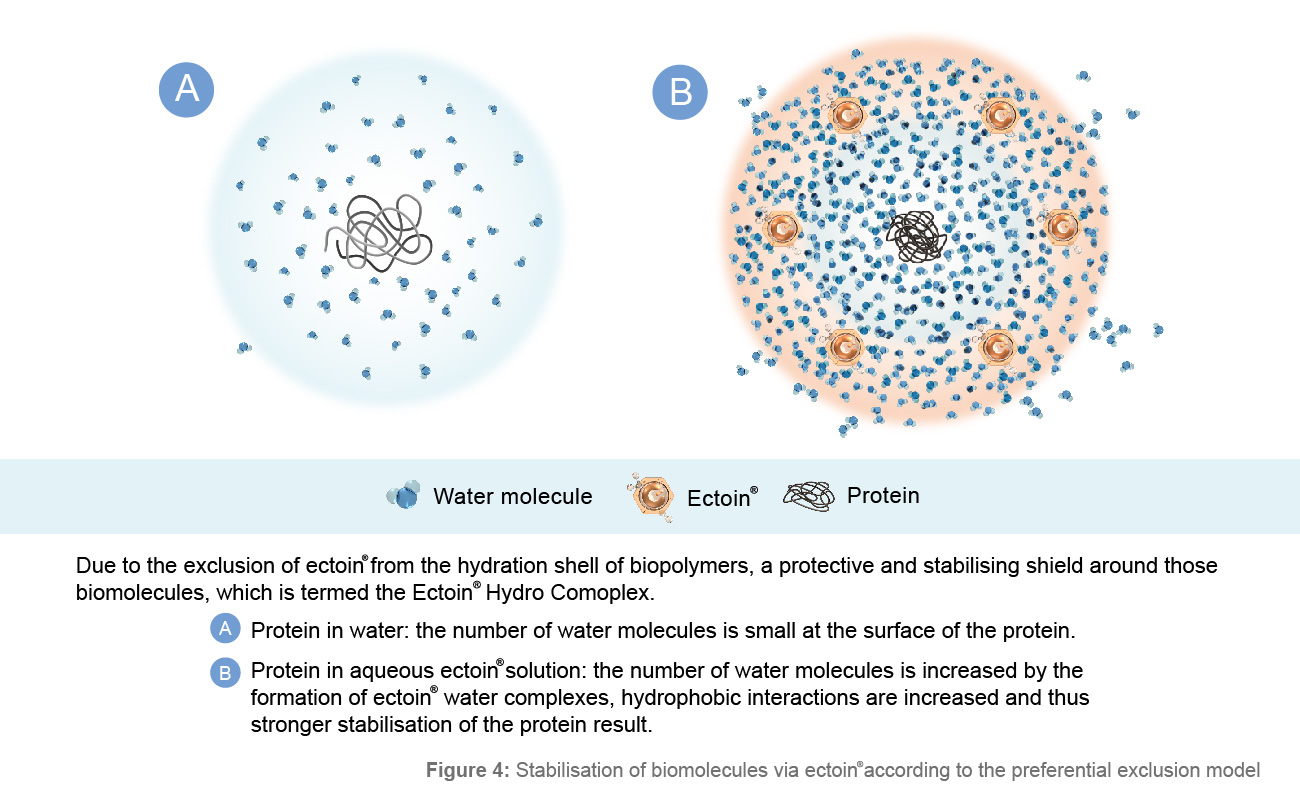
Figure 4: Stabilisation of biomolecules via Ectoin® according to the preferential exclusion model
Due to the exclusion of Ectoin® from the hydration shell of biopolymers, a protective and stabilizing shield is shaped around those biomolecules, the Ectoin® Hydro Complex. The formation of Ectoin® water complexes and thus the kosmotropic effect of Ectoin® on the water structure shown above can stabilize lipid mono- and bilayers, which can be considered a model for cell membranes.
2.Membrane stabilization and increase in membrane fluidity due to Ectoin®
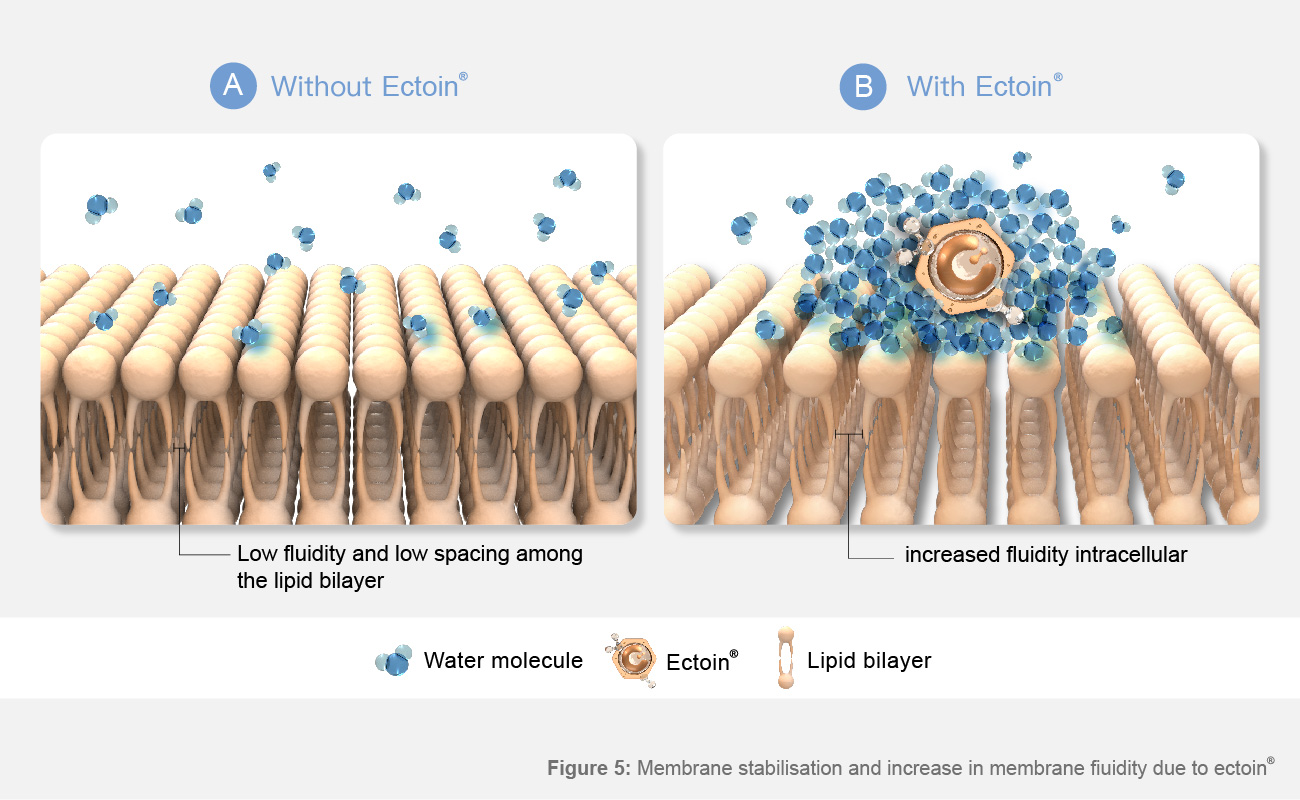
Figure 5: Membrane stabilization and increase in membrane fluidity due to Ectoin®
A:Lipid bilayer in water: The bilayers are stabilized by hydrophilic interactions within head groups.B:Lipid bilayer in aqueous Ectoin® solution: Ectoin® water complexes cause increased interactions of head groups with water, increasing membrane fluidity.
As shown in Figure 3, a lipid bilayer in water is stabilized by hydrophobic interactions of the apolar lipid tail and hydrophilic interactions of the polar lipid head groups to water. In an Ectoin® solution, the hydrophilic interactions are increased by the Ectoin® water complexes, resulting in increased mobility of lipids and thus fluidity of lipid bilayers.
Film balance measurements of lipid monolayers (Figure 4) recently showed the effect of Ectoin® on the fluidity of lipid membranes. Increasing the surface pressure on a DPPC lipid monolayer in water allows the formation of rigid, well-shaped domains at higher pressure. These rigid domains are much smaller in Ectoin® solutions. The higher the concentration of Ectoin®, the smaller the rigid domains. The effect is already observed at the lowest concentration tested (1 mM).
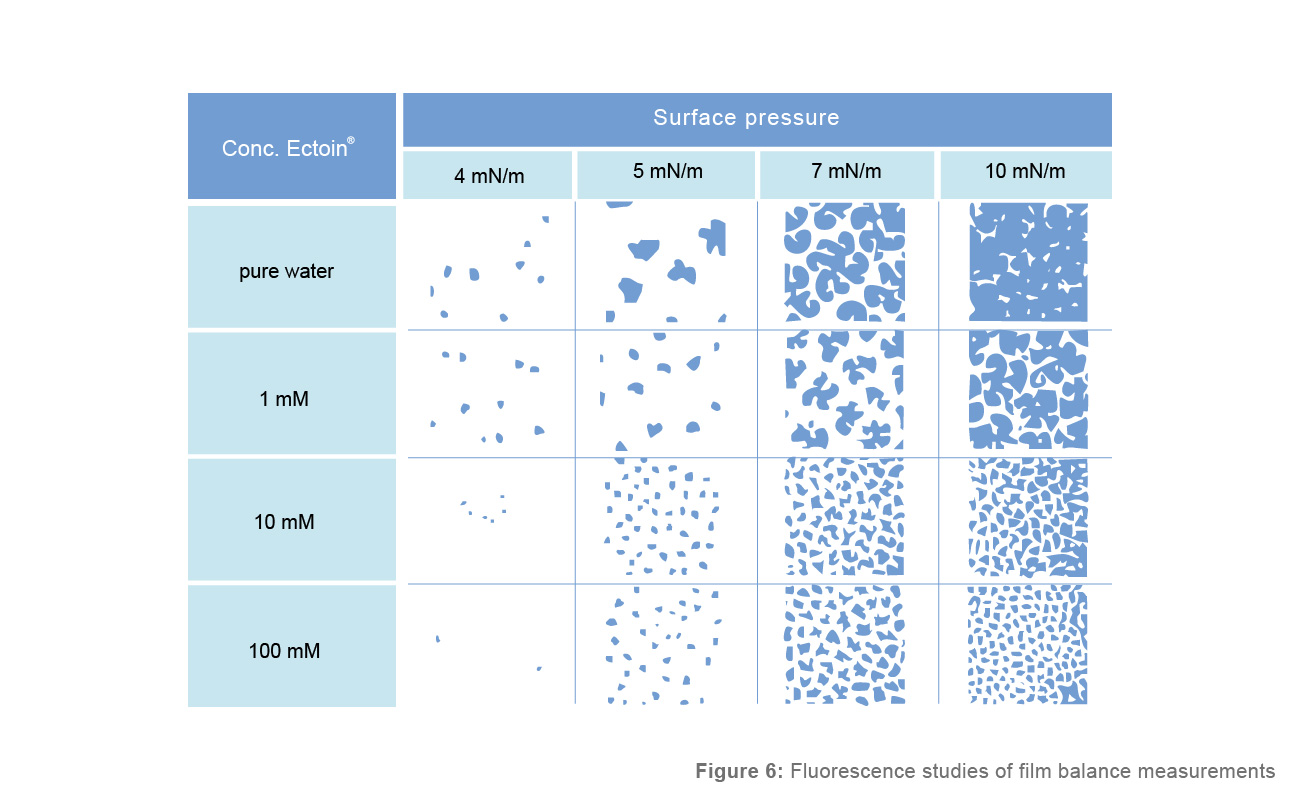
Figure 6: Fluorescence studies of film balance measurements
DPPC (Dipalmitoylglycerophosphatidylcholine) Monolayer at different surface pressure and Ectoin® concentrations. Rigid domains appear black, and fluid domains appear bright. Without Ectoin® and a surface pressure of 10mN/m, the lipid layer of rigid domains exists almost wholly. With Ectoin®, more fluid domains are visible. This effect increases with increasing Ectoin® concentration.
The physical state of the membrane influences cell biology and behavior in response to external inputs. Increased fluidity of the membrane may, for example, induce the expression of stress-responsive, cell-protecting genes, such as heat shock proteins, and reduce ongoing inflammatory processes. Modifying the distribution of membrane proteins in a more fluid membrane also alters their activity.
Increasing membrane fluidity could reduce disease symptoms and accelerate healing. It is crucial for the efficient closure of wounds, and it has been suggested as a mechanism for the very early effects of corticosteroids in asthma therapy and the beneficial effect of dietary moderate ethanol and polyunsaturated fatty acids intake in inflammatory diseases such as psoriasis, allergy, asthma, and inflammatory bowel disease.
3.Prevention of release of stress mediators
Ectoin® stabilizes extracellular membrane components (transmembrane proteins, lipids, extracellular matrix) in their native form. External and internal noxa can cause increased stress for cell membranes without protective mechanisms. Cells that are in direct contact with the environment, like squamous epithelial cells, i.e., skin, upper airway, lung, and intestinal tract, are particularly endangered. The external stress leads to membrane damage, which causes water loss and in- flammatory reactions in the tissue.
The Ectoin® Hydro Complex protects the cells against dehydration by accumulating water. Water molecules are bound more effectively near the membranes and form a stabilising and protecting complex. The impact of external pollutants on the cells is decreased by the stabilising effect of the Ectoin® Hydro Complex. It protects the cells from inflammation caused by environmental stress factors like dehydration, UV radiation, tensides or airborne particles.
Picture 7 : Prevention of release of stress mediators (e.g. ceramides) by Ectoin®.
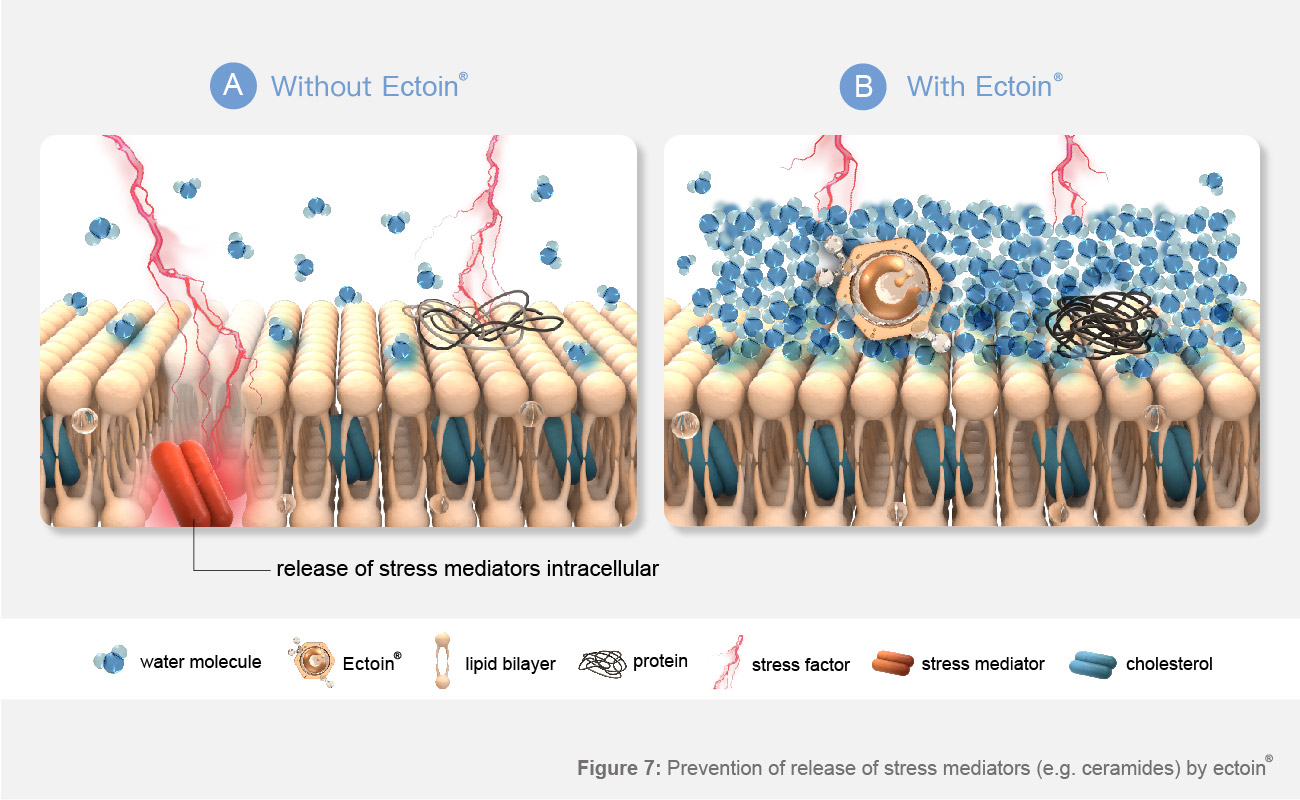
A:Without Ectoin® : external stress factors (like UV irradiation) cause membrane damage, water loss and release of stress mediators, which act as second messengers for infiammatory reactions.B:With Ectoin® : the Ectoin® Hydro Complex protects the membrane against external stress factors; stress mediator releasse is prevented.
Ectoin® helps skin resist external damage
Ectoin® natural is a highly hydrophilic substance with cosmotropic properties, which can increase the number of adjacent water molecules and strengthen the cross-linking structure of water. It stabilizes and protects biomolecules (such as proteins, peptides, enzymes.) and also stabilizes and protects cell membranes. It has extensive, practical uses and product applications in the medical and cosmetic markets. This product has the highest purity specifications in the market, with multiple clinical trials and certifications such as ECOCERT COSMOS and NATRUE international natural standards, providing customers with the best quality products and complete technical services.
Ectoin® repairs particles, which can form an efficient barrier to resist the invasion of foreign molecules. In addition, it is added with ingredients such as “Symcalmin,” an oat extract developed in Germany. It is free of steroids, artificial colors, fragrances, and preservatives, suitable for long-term use. It is ideal for all age groups, including infants aged 28 days, and can be applied around the eyes without irritation, relieving itching.



